Structure-based Design of Drugs for Trypanosomiasis and Leishmaniasis
- Dr. Paul Michels: Molecular Biology
- Dr. Wim Hol: Crystallography
- Dr. Christophe Verlinde: Drug Design
- Dr. Mike Gelb: Synthesis & Enzyme Inhibition Studies
- Dr. Wes van Voorhis and Dr. Fred Buckner: Parasitology
- Dr. Fred Opperdoes: Parasitology
The World Health Organization estimates that 400,000 people are infected
with African trypanosomiasis, with 60 million living in endemic areas.
The disease, caused by the protozoon Trypanosoma brucei and transmitted
by the tse-tse fly, is always fatal without treatment. There is
no vaccine, and current drugs are higly toxic, require administration in
a hospital setting, and are not always effective due to resistance. New
drugs are desperately needed.
More info at WHO:
African trypanosomiasis.
Chagas' disease aka. American trypanosomiasis
The World Health Organization estimates that 18 million people are infected
with Chagas' disease, with 100 million living in endemic areas. The disease,
caused by the protozoon Trypanosoma cruzi and transmitted by reduviid
bugs or via bloodtransfusion, is characterized by an acute transient stage
followed after several years by a chronic stage in a third of the cases.
During the chronic disease irreversible damage to the heart, oesophagus
and colon develops, invariably leading to death, and there are no drugs
to stop this process.
More info at WHO:
Chagas' disease.
Leishmaniasis
Leishmaniasis, caused by eleven species of Leishmania, currently
affects some 12 million people with 350 million at risk, according to the
World Health Organization. There are four forms of the disease. Visceral
leishmaniasis is the most serious form and fatal if untreated. Other forms
are cutaneous, mucocutaneous, and diffuse cutaneous leishmaniasis. All
are accompanied by some of the most horrible tissue destruction and scars
known to mankind.
More info at WHO:
Leishmaniasis.
- Aldolase from T. brucei
- Chudzik D.M., Michels P.A., de Walque S., Hol W.G.J.. (2000). Structures of Type 2 Peroxisomal Targeting Signals in Two Trypanosomatid Aldolases. J Mol Biol. 300, 697-707.
- Triosephosphate isomerase from T. brucei
- Wierenga R.K., Noble M.E., Vriend G., Nauche S., Hol W.G.J. (1991). Refined 1.83 A structure of trypanosomal triosephosphate isomerase crystallized in the presence of 2.4 M-ammonium sulphate. A comparison with the structure of the trypanosomal triosephosphate isomerase-glycerol-3-phosphate complex. J Mol Biol. 220, 995-1015.
- Glyceraldehyde-3-phosphate dehydrogenase from T. brucei and L. mexicana
- Vellieux F.M.D., Hajdu J., Verlinde C.L.M.J., Groendijk H., Read R.J., Greenhough T.J., Campbell J.W., Kalk K.H., Littlechild J.A., Watson H.C., Hol W.G.J. (1993). Structure of glycosomal glyceraldehyde-3- phosphate dehydrogenase from Trypanosoma brucei determined from Laue data. Proc. Natl. Acad. Sci. USA 90, 2355-2359. [MEDLINE: 8460146].
- Kim H., Feil I.K., Verlinde C.L.M.J., Petra P.H., Hol W.G.J. (1995). Crystal structure of glycosomal glyceraldehyde-3-phosphate dehydrogenase: from Leishmania mexicana: Implications for structure- based drug design and a new position for the inorganic phosphate binding site. Biochemistry 34, 14975-14986. [MEDLINE: 7578111]
- Phosphoglycerate kinase from T. brucei
- Bernstein BE, Michels PAM, Hol WGJ (1997). Synergistic effects of substrate- induced conformational changes in phosphoglycerate kinase activation. Nature 385, 275-278.
- Bernstein BE, Williams DM, Bressi JC, Kuhn P, Gelb MH, Blackburn GM, Hol WGJ (1998). A bisubstrate analog induces unexpected conformational changes in phosphoglycerate kinase from Trypanosoma brucei. J Mol Biol 279, 1137-1148.
- Glycerol-3-phosphate dehydrogenase from L. mexicana
- Suresh S., Turley S., Opperdoes F.R., Michels P.A., Hol W.G.J. (2000). A potential target enzyme for trypanocidal drugs revealed by the crystal structure of NAD-dependent glycerol-3-phosphate dehydrogenase from Leishmania mexicana. Structure Fold Des. 8, 541-52.
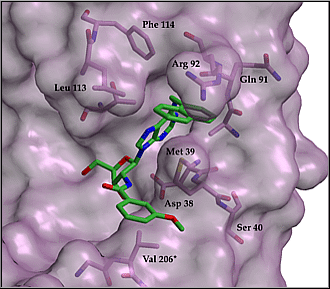
Fig. 1: Predicted binding mode of designed inhibitor.
The inhibitor has also been tested on cultures of live trypanosomes. In vitro it blocks the growth of bloodstream form T. brucei with an ED50 of about 30 µM. In addition the compound was tested for its ability to inhibit the growth of T.cruzi amastigotes in T3T fibroblast host cells. Similar effects as in T.brucei were seen, and the compound appeared to be harmless to mammalian cells at concentrations up to 0.05 mM.
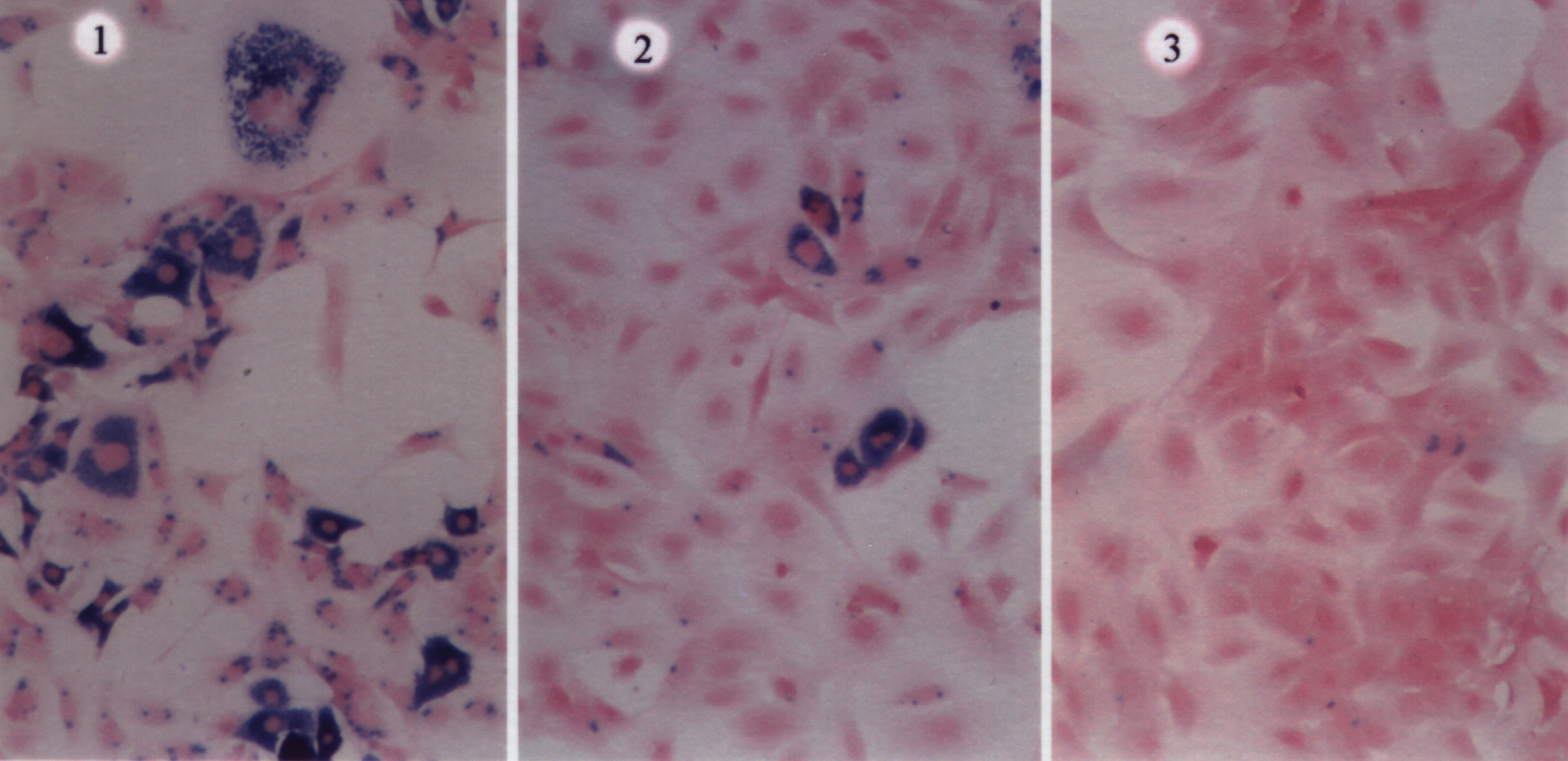
Fig. 2: Optical microscopy showing T3T fibroblasts infected with
T.cruzi in absence of inhibitor (pannel 1), in presence of 16 µM(pannel
2), and of 40 µM inhibitor (pannel 3).